Systemic Inflammation: The Hidden Trigger of Chronic Diseases
1) Introduction
SIRS is an excessive defense reaction of the
body to a noxious stressor such as infection, trauma, surgery, acute
inflammation, ischemia or reperfusion, or cancer, among others. The goal of
this response is to identify and then eradicate the cause of the insult,
whether endogenous or exogenous. It is characterized by the release of
acute-phase reactants, which function as direct mediators of broad autonomic,
endocrine, hematological, and immunological changes in the individual. The
etiopathogenesis of systemic inflammatory response syndrome is broadly divided
into Damage Associated Molecular Pattern (DAMP) and Pathogen Associated
Molecular Pattern (PAMP) at the molecular level.
SIRS is determined by vital signs and a
leukocyte count. However, the stress of arriving at a healthcare facility in
extremes of age or the concurrent use of drugs might cause these signs to be
incorrectly altered. As a result, establishing the diagnosis requires periodic
examination of vital signs and evidence of persistent instability. The
identification of the continuum from early inflammation to multiorgan
dysfunction has added more incentive to the definition of SIRS over time. This
has resulted in the need to detect SIRS both in the context of infection and in
noninfectious stress, where the body becomes vulnerable to secondary infection.
b) Epidemiology
of Systemic Inflammatory Response Syndrome (SIRS)
Systemic Inflammatory Response Syndrome (SIRS)
is a common disorder, especially in hospitals. It affects one-third of all
in-hospital patients and more than half of all ICU patients. SIRS is more
common among surgical ICU patients, where it affects more than 80% of patients.
Trauma patients are more vulnerable to SIRS,
and the majority of these patients do not have a proven infection. The
prevalence of infection and bacteremia (the presence of bacteria in the blood)
rises in direct proportion to the number of SIRS criteria met and the severity
of the septic symptoms.
About one-third of SIRS patients progress to sepsis, a potentially fatal illness caused by the body's response to an infection. Sepsis affects roughly 25% of ICU patients, with bacteremic sepsis affecting 10%. The frequency of SIRS among surgical ICU patients is significantly higher, with 93% of patients satisfying the SIRS criteria. The SIRS score, which is used to evaluate the severity of the syndrome, falls by 0.8 points on average from the first to second day of the ICU stay, reflecting the effect of ICU resuscitation.
SIRS development throughout the ICU stay is associated with an increase in the incidence of multiple organ dysfunction (MOD), longer hospital stays, and increased death. In one study, 78% of patients with significant trauma got SIRS, while 72.5% had MODS (Multiple Organ Dysfunction Syndrome). The development of SIRS and MODS was strongly related with mortality, with 23% of patients who had SIRS on day 1 dying compared to 6.8% of individuals who did not have SIRS on day 1.
Finally, SIRS is a prevalent and dangerous
condition in hospital settings, notably in intensive care units and among
trauma victims. It is related with an increased risk of developing sepsis and
MODS, as well as a considerable increase in mortality among affected
individuals. As a result, early detection and therapy of SIRS are critical for
improving patient outcomes.
c) Importance
of Collaboration and Communication in Managing SIRS
Collaboration and communication among the
interprofessional team are required for effective SIRS management. This is
because teamwork among physicians, nurses, and other health care professionals
raises team members' awareness of each other's knowledge and skills, resulting
in ongoing decision-making improvement. Trust, respect, and collaboration
characterize effective teams. In the setting of SIRS, teamwork is critical in
developing and implementing patient care plans, ensuring adherence to treatment
standards, and improving the clinical outcome of systemic inflammatory response
syndrome.
2) Etiopathogenesis of Systemic Inflammation
Infection, trauma, surgery, acute inflammation,
ischemia or reperfusion, or cancer are all examples of noxious stressors that
cause systemic inflammation. The goal of this response is to identify and then
eradicate the cause of the insult, whether endogenous or exogenous. It is
characterized by the release of acute-phase reactants, which function as direct
mediators of broad autonomic, endocrine, hematological, and immunological
changes in the individual. The dysregulated cytokine storm, on the other hand, can
trigger a huge inflammatory cascade, leading to reversible or irreversible
end-organ damage and even death. A manifestation of this systemic inflammation
is systemic inflammatory response syndrome (SIRS). Sepsis is defined as SIRS
with a probable source of infection, while severe sepsis is defined as sepsis
with one or more end-organ failures. Septic shock is defined as hemodynamic
instability despite intravascular volume repletion.
a) Damage
Associated Molecular Pattern (DAMP)
DAMPs are endogenous danger compounds that are
generated by injured or dying cells and activate the innate immune system
through interactions with pattern recognition receptors (PRRs). Although DAMPs
aid in host defense, they also trigger pathological inflammatory responses. In
inflammatory illnesses, DAMPs such as high-mobility group box 1 (HMGB1), S100
proteins, and heat shock proteins (HSPs) are elevated and thought to play a
pathogenic function. Extracellular proteins, such as biglycan and tenascin C, and
intracellular proteins, such as HMGB1, histones, S100 proteins, heat-shock
proteins (HSPs), and plasma proteins, such as fibrinogen, Gc-globulin, and
serum amyloid A (SAA), are examples of DAMPs.
b) Pathogen
Associated Molecular Pattern (PAMP)
Pathogen-associated molecular patterns (PAMPs)
are tiny molecular motifs that are shared among a class of microorganisms but
not found in the host. The immune system recognizes these chemicals and
initiates an immunological response. In both plants and animals, PAMPs are
detected by toll-like receptors (TLRs) and other pattern recognition receptors
(PRRs), allowing the innate immune system to recognize pathogens and defend the
host against infection.
PAMPs are a diverse group of compounds that
include glycans, glycoconjugates, and proteins such as flagellin. PAMPs include
nucleic acid variations associated with viruses, such as double-stranded RNA
(dsRNA). Bacterial lipopolysaccharides (LPSs), commonly known as endotoxins,
are endotoxins present on the cell membranes of gram-negative bacteria and are
thought to constitute the prototype family of PAMPs.
When PAMPs are recognized by PRRs, multiple
signaling cascades in the host immune cells are activated, increasing the
production of interferons (IFNs) or other cytokines. Through the generation of
different inflammatory cytokines, chemokines, and type I interferons, this
process establishes intricate interactions between the pathogen and the host,
rapidly unleashing a variety of anti-microbial immune responses.
PAMPs are required for microbial survival and
pathogenicity. They are detected by pathogen recognition receptors (PRRs),
which are germline-encoded host sensors. Toll-like receptors (TLRs), RIG-I-like
receptors (RLRs), NOD-like receptors (NLRs), and DNA receptors (cytosolic
sensors for DNA) are all recognized to play important roles in host defense.
The word "PAMP" has been questioned
because most bacteria, not just pathogens, express the chemicals discovered; as
a result, the term microbe-associated molecular pattern (MAMP) has been
proposed. A (pathogen-specific) PAMP has been proposed as a virulence signal
capable of binding to a pathogen receptor in combination with a MAMP.
PAMPs, in summary, are critical components of
the immune response, allowing the host organism to recognize and respond to
pathogens. They are identified by certain immune system receptors, resulting in
a cascade of immunological responses that assist to protect the host from
infection.
c) Common
Etiologies from a Clinical Perspective
Chronic inflammatory illnesses are the leading
cause of death worldwide. Chronic diseases are ranked as the greatest hazard to
human health by the World Health Organization (WHO). In the United States, the
prevalence of disorders related with chronic inflammation is expected to rise
steadily over the next 30 years. Chronic inflammatory diseases such as stroke,
chronic respiratory diseases, heart issues, cancer, obesity, and diabetes kill
three out of every five individuals worldwide. Chronic inflammation is linked
to a variety of disorders, including cardiovascular disease, diabetes, cancer,
auto-immune disease, chronic hepatic and renal disease, and others. Chronic
inflammation that goes untreated has a terrible prognosis. The causal
mechanistic pathway that leads to chronic inflammation determines
disease-specific morbidity and mortality.
3) Mechanisms of Systemic Inflammation
a) Role of
Humoral and Cellular Immune Response
The human immune system is a complicated system
that includes both humoral and cellular components. The humoral immune response
is predominantly mediated by B cell-produced antibodies, whereas the cellular
immune response involves many cell types that recognize and destroy pathogens
and cellular debris.
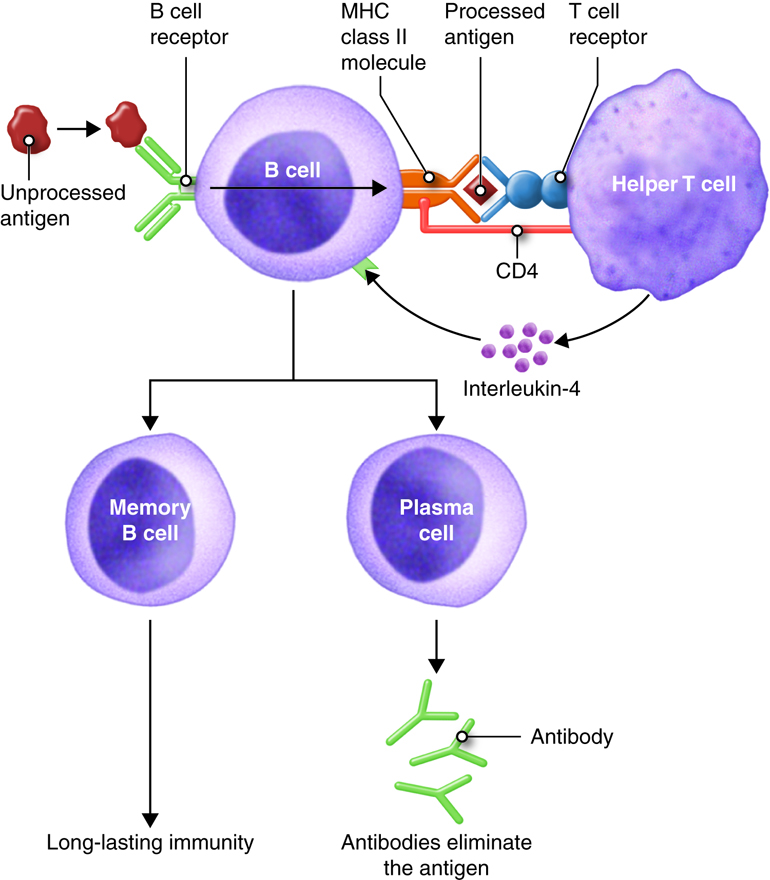
i) Humoral
Immune Response
Antibody molecules released by plasma cells, a
kind of B cell, mediate the humoral immune response. Antibodies are created in
reaction to antigens, which are substances recognized by the immune system as
foreign. Antigen stimulates B cell activation and differentiation into
antibody-secreting plasma cells, which normally necessitates the involvement of
helper T cells, a kind of immune cell that aids in the immunological response.
Antibodies help with immunity in three ways.
Pathogens can be neutralized by attaching to them and blocking them from
entering cells. They can also aid phagocytic cell uptake of pathogens, a
process known as opsonization. Finally, antibodies can activate complement
system proteins, resulting in pathogen elimination.
Natural antibodies (NAb), pentraxins, and the
complement and contact cascades are all components of the humoral innate immune
response. These components are critical in disease prevention and control.
Pathogens and cells with altered self proteins, on the other hand, can use
numerous humoral components to avoid elimination and induce disease.
Natural antibodies are produced by a subset of
B lymphocytes, primarily B1 cells and B lymphocytes from the marginal zone.
They are the first line of defense against infections, prior to the formation
of germinal centers, which produce adaptive antibodies. They are found in many
animals, including humans, and are made up mostly of immunoglobulin M,
immunoglobulin A (IgA1 and IgA2), and IgG, primarily IgG3, but also IgG1, IgG2,
and IgG4.
Together with the complement system, pentraxins
such as C-reactive protein (CRP), serum amyloid protein P (SAP), and
pentraxin-3 (PTX3) coordinate geographically and temporally targeted clearance
of wounded tissue components, defend against infections, and regulate related
inflammation. They have a symbiotic connection with the complement system,
activating it after binding to their targets but suppressing it at the C3b
stage to prevent over-damage.
ii) Cellular
Immune Response
The cellular component of the immune response
includes a variety of cell types that recognize and eliminate pathogens and
cellular debris using pattern recognition molecules. Pattern Recognition
Receptors (PRRs) are proteins that recognize compounds contained in infections
(known as Pathogen-Associated Molecular Patterns—PAMPs) or molecules generated
by damaged cells (known as Damage-Associated Molecular Patterns—DAMPs). They
appeared phylogenetically prior to the advent of adaptive immunity and are thus
regarded to be part of the innate immune system.
Finally, both the humoral and cellular immune
responses are important in the body's defense against infections. The humoral
response entails the generation of antibodies that can kill infections, aid in
their absorption by immune cells, or trigger the complement system to destroy
them. Pathogens and cellular detritus are recognized and removed by numerous
immune cells throughout the cellular response. Understanding these pathways is
critical for creating effective treatments for infectious illnesses and other
immune-related ailments.
b) Role of
Cytokines and Complement Pathway
i) Role of Cytokines in Systemic Inflammatory Response
Cytokines are tiny proteins that play an
important function in the inflammatory response in the body. They are released
during the inflammatory response and, if their release is disrupted, can create
a large inflammatory cascade. Cytokines are involved in a variety of functions,
including cell signaling, immune cell activation, and inflammatory regulation.
They are divided into pro-inflammatory and anti-inflammatory cytokines, which
stimulate and decrease inflammation, respectively.
Tumor necrosis factor-alpha (TNF-),
interleukin-1 (IL-1) and interleukin-6 (IL-6) are pro-inflammatory cytokines
produced by immune cells such as macrophages and are involved in the start and
amplification of the inflammatory response. These cytokines have the capacity
to induce the production of other inflammatory mediators, attract immune cells
to the site of inflammation, and enhance vascular permeability, allowing immune
cells to reach the affected area.
Anti-inflammatory cytokines such as
interleukin-10 (IL-10) and interleukin-4 (IL-4) aid in balancing the
pro-inflammatory response and preventing excessive inflammation. They have the
ability to block the generation of pro-inflammatory cytokines, reduce immune
cell activation, and facilitate inflammation resolution.
ii) Role of
Complement Pathway in Systemic Inflammation
The complement system is an important component
of the innate immune response because it protects the host from infections and
aids in the repair of damaged tissues. It is made up of a series of proteins
that are triggered by a proteolytic cascade, resulting in the production of
complement effectors that target diverse immune cells.
There are three recognized complement
activation pathways: classical, lectin, and alternative. The activation of the
core component C3, which is cleaved into C3a and C3b, brings all three routes
together. C3b has the ability to bind to pathogens and assist their
phagocytosis, whereas C3a works as an anaphylatoxin, causing inflammation and
attracting immune cells to the site of infection.
To influence the inflammatory response, the
complement system interacts with other immunological components such as
cytokines and immune cells. The anaphylatoxins C3a and C5a, for example, can
increase the release of pro-inflammatory cytokines, so boosting the
inflammatory response.
c) Balance
between Proinflammatory and Anti-inflammatory Cascades
A critical element of the systemic inflammatory
response is the balance of proinflammatory and anti-inflammatory responses.
This equilibrium is dynamic, requiring constant feedback from both the host and
the pathogen. It can be accomplished in a variety of ways, including
interactions between pro- and anti-inflammatory cytokines and cells at the
molecular, organ, and whole-host levels. This equilibrium is not necessarily
quantitative, but rather a qualitative balance between downstream activation
and inhibition.
In the case of rheumatoid arthritis, the
disease is caused by immune system imbalances caused by an accumulation of
environmental and behavioral assaults throughout a lifetime, mixed with
hereditary predispositions. The inflammatory reactions that cause such
disorders are typically triggered by autoimmune responses against normal,
modified, or immuno-mimetic self-proteins found in skeletal joints.
Systemic Inflammatory Response Syndrome (SIRS)
is characterized by dysregulation of proinflammatory and antiinflammatory
pathway homeostasis, as well as dysregulated release of acute and chronic phase
reactants. This syndrome is caused by the body's excessive defense reaction to
a noxious stressor in order to pinpoint and then eradicate the endogenous or
external source of the insult. The dysregulated cytokine storm, on the other
hand, can trigger a huge inflammatory cascade, leading to reversible or irreversible
end-organ damage and even death.
The aberrant inflammatory response is closely
connected with many chronic disorders in autoimmune diseases. T cell-mediated
inflammatory responses, including Th1, Th2, and Th17 cell responses, have long
been recognized as critical in the development of autoimmune disorders.
Abnormal T cell immune responses, including Th1, Th2, and Th17 cell responses,
play a critical role in autoimmune disease inflammation.
Post-translational modification (PTM) proteins
can activate autoimmune reactions and change the normal balance of immunity in
the presence of systemic inflammation, which can be increased by trauma,
infection, or other inflammatory events. Exposure to pathogen-associated
molecular pattern molecules (PAMPs) of microbes or damage-associated molecular
pattern molecules (DAMPs) induced by tissue damage or trauma can activate
processes that initiate inflammatory responses to PTM self-(auto)antigens that
drive diseases like rheumatoid arthritis.
To summarize, it is critical for the body's
immune system to maintain a balance between proinflammatory and
anti-inflammatory responses. Disruptions in this balance can result in a
variety of diseases, including autoimmune disorders such as rheumatoid
arthritis. Understanding the processes that regulate this equilibrium has the
potential to yield considerable clinical advantages in the treatment of various
disorders.
4) Diagnosis of
Systemic Inflammation
The assessment of vital signs, particularly
body temperature, heart rate, and breathing rate, is critical in the diagnosis
of Systemic Inflammatory Response Syndrome (SIRS). SIRS is an increased body's
defense reaction to a damaging stressor, which can cause significant
inflammation throughout the body, potentially leading to reversible or
irreversible organ failure and even death.
In a study of adult out-of-hours (OOH) primary
care patients with suspected infections, at least two SIRS vital signs were
found to be abnormal in 8.6% of clinic consultations and 40.3% of home visits.
SIRS criteria were a temperature of 36 or greater than 38 °C, a heart rate of
more than 90 beats per minute, and a respiratory rate of more than 20 breaths
per minute.
When no SIRS vital sign was abnormal, the
referral rate increased from 13% to 68% when all three SIRS vital indicators
were abnormal. This suggests that the presence of aberrant SIRS vital signs can
have a considerable impact on the decision to refer a patient for additional
treatment. It should be noted, however, that particular SIRS vital signs were
not independently related with hospital referral.
Instead, it was shown that decreased oxygen
saturation, hypotension, and rapid illness progression were more important in
directing future care. This shows that, while SIRS vital signs can be useful,
other clinical indications and symptoms may be more important in assessing the
necessity for hospitalization.
It's also worth noting that vital sign
examination is part of a complicated diagnostic process in primary care. Aside
from vital signs, several other components of the consultation influence the
decision to refer a patient to the hospital. As a result, while assessing SIRS
vital signs is important, it should be done as part of a larger clinical
assessment.
To summarize, evaluating SIRS vital signs is an
important element of diagnosing SIRS and guiding subsequent care. Other
clinical signs and symptoms, such as decreased oxygen saturation, hypotension,
and rapid illness progression, may, however, be more essential in determining
the necessity for hospitalization.
b) Role of
Biomarkers in Diagnosis
Biomarkers are important in the diagnosis and
treatment of systemic inflammation. They serve as the foundation for disease
diagnosis, drug discovery, and disease monitoring. Biomarkers produced from
body fluids offer considerable potential for optimizing patient therapy in the
setting of chronic inflammatory disorders.
In patients with inflammatory bowel diseases
(IBD), such as Crohn's disease (CD) or ulcerative colitis (UC), for example,
several laboratory markers have been investigated for diagnosis and
differential diagnosis of IBD, as well as assessment of disease activity and
risk of complications, prediction of relapse, and monitoring the effect of
therapy. These biomarkers are classified as serological, fecal, or other
biomarkers.
i) Serological
Biomarkers
Serological biomarkers are detectable compounds
in bodily fluids (blood) whose use is less expensive, less laborious, less
invasive, and more objective than endoscopy/biopsy. Acute-phase reactants,
cytokines, and other substances are among them.
C-reactive protein (CRP) and erythrocyte
sedimentation rate (ESR) are frequent acute-phase reactants used in the
diagnosis and monitoring of inflammatory disorders. They are made by the liver
in response to inflammation and can provide important information regarding the
existence and degree of inflammation in the body.
Tumor necrosis factor-alpha (TNF-),
interleukin-1 (IL-1) and interleukin-6 (IL-6) are also relevant serological
indicators. They have a role in immune response control and can be utilized to
measure the intensity of inflammation and track therapy response.
ii) Fecal
Biomarkers
Fecal biomarkers are useful because they are
unique to the gastrointestinal system. They are a diverse range of chemicals
that either leak from or are produced by irritated intestinal mucosa.
Fecal calprotectin, a protein generated by
neutrophils during inflammation, is one of the most extensively utilized fecal
indicators. Fecal calprotectin levels are higher in IBD patients and can be
used to distinguish IBD from irritable bowel syndrome (IBS), as well as to
monitor disease activity and response to treatment.
Lactoferrin and S100A12 are two more fecal
biomarkers that have been studied for their potential use in the diagnosis and
management of IBD.
Finally, biomarkers are critical in the
diagnosis and management of systemic inflammation, particularly in chronic
inflammatory disorders. Serological and fecal biomarkers provide useful
information regarding the presence and intensity of inflammation, enabling for
better patient management and therapy response monitoring.
5) Complications
of Systemic Inflammation
a) Progression
to Sepsis, Severe Sepsis, Shock, and Multiorgan Dysfunction Syndrome
Sepsis, a systemic inflammatory response
syndrome (SIRS) caused by either viral or non-infectious causes, can develop
from systemic inflammation. Sepsis, if not treated promptly, can progress to
septic shock and multiple organ dysfunction syndromes (MODS), with a death rate
of 28-56%.
Sepsis is caused by bacterial toxins activating
inflammatory response cells, resulting in the release of inflammatory mediators
such as interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-
(TNF-). This causes systemic or local inflammatory responses, which result in
symptoms such as mental disturbances, shortness of breath, palpitation, fever,
and chills.
Patients suffering from sepsis as a result of
shock, infection, trauma, or other causes may experience pathophysiological
changes in their circulatory, respiratory, and nervous systems, such as tissue
and organ ischemia and hypoxia, renal function decline, electrolyte
disturbance, shortness of breath, blood pressure drop, and acid-base imbalance.
MODS and even mortality may result if these early signs are not treated.
End-organ dysfunction is a serious consequence
of systemic inflammation in which a dysregulated cytokine storm can set off a
huge inflammatory cascade that results in reversible or irreversible end-organ
malfunction and even death.
Severe sepsis is defined as sepsis with one or
more end-organ failures, and septic shock is defined as hemodynamic instability
despite intravascular volume repletion. The presence of altered organ function
in acutely unwell septic patients such that homeostasis is not maintainable
without intervention is defined as multiple organ dysfunction syndrome (MODS).
The Acute Physiology and Chronic Health
Evaluation (APACHE) score version II and III, Multiple organ dysfunction (MOD)
score, sequential organ failure assessment (SOFA), and logistic organ
dysfunction (LOD) score are all used to determine the severity of organ system
damage.
Finally, uncontrolled systemic inflammation can
result in severe complications such as sepsis, severe sepsis, shock, and
multiorgan dysfunction syndrome, which can result in end-organ dysfunction and
even death. To avoid these consequences, early detection and treatment of
systemic inflammation are critical.
6) Management
of Systemic Inflammation
a) Treatment
Guidelines for Systemic Inflammation
Systemic inflammation treatment focuses on
addressing the underlying cause. Rest, ice, and excellent wound care can
frequently reduce pain from acute inflammation. Chronic inflammation, on the
other hand, may necessitate a more complete therapy. Certain vitamins (A, C, D)
and supplements (such as zinc) may help to minimize inflammation and repair.
Anti-inflammatory spices such as turmeric, ginger, and garlic may also be
useful. Inflammation can be reduced with over-the-counter medications such as
ibuprofen, aspirin, or naproxen. Corticosteroid injections alleviate
inflammation in a specific joint or muscle. However, no more than three to four
injections in the same body area per year are recommended. An anti-inflammatory
diet, such as the Mediterranean diet, can reduce inflammation levels. This
includes eating more anti-inflammatory foods such as oily salmon, leafy greens,
olive oil, and tomatoes while avoiding foods that induce inflammation such as
fried foods, cured meats with nitrates, highly processed oils and trans fats,
and refined carbohydrates. Maintaining a healthy weight, avoiding or stopping
smoking, exercising frequently, limiting alcohol intake, and managing stress
are all good practices that help reduce the risk of chronic inflammation.
Antibiotics such as oritavancin, dalbavancin, and tedizolid can be used to
treat acute bacterial skin and skin structure infections in patients with
systemic inflammatory response syndrome (SIRS). Although steroids have been
extensively investigated for sepsis and septic shock, no studies on systemic
inflammatory response syndrome (SIRS) have been conducted to yet. Low-dose
steroids (200-300 mg hydrocortisone for 5-7 days) increased survival and shock
reversal in vasopressor-dependent patients, according to studies.
b) Role of
Interprofessional Team
An interprofessional team's involvement in controlling systemic inflammation is diverse and extends beyond medical care. This multidisciplinary team, which may include primary care physicians, nurses, physical therapists, dieticians, and specialists, collaborates to improve care coordination and communication, with the ultimate goal of controlling chronic inflammation and improving patient outcomes. Patient education is a critical component of the interprofessional team. The team educates patients on the importance of lifestyle changes, dietary alterations, and medication adherence in treating systemic inflammation. This teaching role is critical because it encourages patients to take an active role in their own care, which can result in improved health outcomes.
Dentists and dental hygienists, for example,
can also be valuable members of interprofessional teams. Because of their work
in areas such as temporomandibular joint dysfunction (TMD) and sleep medicine,
they can make a substantial contribution to the treatment of chronic pain
patients. They can also help with screening patients for certain primary care
metrics and controlling the impact of drugs on oral health.
Interprofessional teams are very important in the treatment of chronic inflammatory arthritis. Nurses, for example, play a significant role in the monitoring and treatment of individuals suffering from this ailment. They educate patients to increase their understanding of the condition and its management, as well as contribute to improved communication, continuity, and satisfaction with care. Interprofessional teams of physiotherapists have been demonstrated to have positive benefits on the treatment of adults with low back pain. They learn new information as providers and feel respected in their roles, which leads to better overall treatment and outcomes. Interprofessional collaboration entails more than just professionals working side by side; it entails a higher level of interaction and cooperation. It necessitates each team member's well-developed and strong professional identity, as well as a shift from a single disease focus to a study of the numerous components of the patient's multimorbidity.
To summarize, the role of an interprofessional
team in the management of systemic inflammation is broad and entails a
collaborative approach to patient care. This includes patient education,
screening, drug impact control, and the provision of specialized treatment. The
concepts of teamwork, communication, and mutual respect guide the team's work,
with the ultimate goal of improving patient outcomes.
7) Organ-Specific
Inflammatory Responses
Inflammation is a biological immunological
reaction that can be produced by a number of reasons such as pathogens, damaged
cells, and toxic substances. These factors have the potential to cause acute
and/or chronic inflammatory reactions in multiple organs, potentially leading
to tissue damage or disease.
Heart inflammation can take numerous forms,
including pericarditis, myocarditis, and endocarditis. These symptoms are
usually the body's response to an infection or damage. Chest pain and shortness
of breath are common symptoms.
b) Inflammation
in the Pancreas
Pancreatitis is an inflammation of the pancreas
that can happen suddenly (acute pancreatitis) or gradually over time (chronic
pancreatitis). This inflammation can result in edema, discomfort, and
abnormalities in pancreatic function. Gallstones are a common cause of
pancreatitis because they block the bile duct and cause pancreatic enzymes to
irritate pancreatic cells, resulting in inflammation. Repeated bouts of acute
pancreatitis can progress to chronic pancreatitis, resulting in complications
such as renal failure, breathing difficulties, infection, malnutrition, and
diabetes.
Inflammation of the liver can be caused by a
number of reasons, including parasitic and viral infection, immune system
abnormalities, genetic problems, and toxicity exposure. Conditions that harm
the liver over time can cause scarring (cirrhosis), which can progress to liver
failure, a potentially fatal condition. Symptoms of liver illness may include
yellow skin and eyes, abdominal pain, and weariness.
Infections, autoimmune illnesses, or exposure
to specific toxins can all cause kidney inflammation, commonly known as
nephritis. Blood in the urine, elevated blood pressure, and swelling in the
hands and feet owing to fluid retention are all possible symptoms.
Infections, allergies, chronic respiratory
illnesses such as asthma and chronic obstructive pulmonary disease (COPD), and
exposure to certain environmental chemicals can all induce lung inflammation.
Shortness of breath, coughing, and chest tightness are all possible symptoms.
Infections, autoimmune illnesses, and certain
drugs can all induce brain inflammation, often known as encephalitis. Headache,
fever, disorientation, and seizures are all possible symptoms.
g) Inflammation
in the Intestinal Tract
Infections, autoimmune illnesses such as
Crohn's disease and ulcerative colitis, and exposure to specific poisons can
all induce intestinal inflammation. Abdominal pain, diarrhea, and weight loss
are all possible symptoms.
h) Inflammation
in the Reproductive System
Infections, hormonal imbalances, and disorders
such as endometriosis in women and prostatitis in men can all induce
inflammation in the reproductive system. Pain during intercourse, irregular
menstruation cycles in women, and erectile dysfunction in men can all be
symptoms.
8) Chronic
Inflammatory Systemic Diseases
Chronic Inflammatory Systemic Diseases (CIDs)
are a set of illnesses marked by long-term inflammation that can last months or
years. Rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis,
and other disorders fall within this category. Humans bear the burden of CIDs
due to life-long severe illness, increased mortality, and expensive therapy and
care costs.
These disorders involve the immune system,
neurological system, endocrine system, and reproductive system. Maladaptations
of these systems occur during CIDs, resulting in disease sequelae. The presence
of the same signaling factors in diverse CIDs suggests that these disorders
have a pathogenesis.
Environmental variables are also important in
the establishment of CIDs. Tobacco use, for example, has been associated to an
increased risk of rheumatoid arthritis, ankylosing spondylitis, multiple
sclerosis, Crohn's disease, and systemic lupus erythematosus. Other
environmental factors, including as alcohol intake, nutrition, and exposure to
certain industrial toxins, can all have an impact on the likelihood of
acquiring these disorders.
a) Sequelae of
Chronic Inflammatory Systemic Diseases
The term "sequelae" refers to the
long-term repercussions or complications of an illness. These sequelae
frequently appear as different health disorders or syndromes in the context of
CIDs. For example, the hypothesis anticipates the emergence of long-term
disease sequelae like metabolic syndrome.
Chronic inflammation can cause severe changes
in all tissues and organs, as well as normal cellular physiology, raising the
risk of numerous noncommunicable diseases in both young and old people. Chronic
inflammation can also affect normal immune function, increasing vulnerability
to infections and malignancies and impairing vaccine response.
b) Role of
Inflammation in Disease Onset or Progression
Inflammation is an evolutionary conserved
process that involves the activation of immune and non-immune cells in order to
defend the host against bacteria, viruses, poisons, and diseases by removing
pathogens and encouraging tissue repair and recovery. Certain social,
psychological, environmental, and biological factors, on the other hand, can
prevent acute inflammation from resolving, fostering a state of low-grade,
non-infective systemic chronic inflammation (SCI).)
Inflammation appears to play a substantial role
in the beginning or progression of diseases, including metabolic syndrome, type
2 diabetes, and cardiovascular disease, according to empirical research. For
example, it has long been known that patients with autoimmune diseases
characterized by systemic inflammation, such as rheumatoid arthritis, have
insulin resistance, dyslipidemia, and hypertension, as well as higher rates of
metabolic syndrome, type 2 diabetes, and cardiovascular disease.
To summarize, chronic inflammatory systemic
disorders are complicated problems involving several bodily systems and
influenced by both genetic and environmental factors. The persistent
inflammation that characterizes these disorders can result in a range of
long-term health problems, emphasizing the necessity of early detection and
treatment.
9) Systemic
Chronic Inflammation (SCI)
Systemic Chronic Inflammation (SCI) is a
gradual, long-term inflammation that can last months or years. It is a
component of the body's defensive mechanism, identifying and eliminating
harmful and foreign stimuli as well as commencing the healing process. When
this inflammation becomes persistent, it can cause a variety of health
problems. Chronic inflammation can be caused by a failure to eliminate the
agent that causes acute inflammation, exposure to a low level of a specific
irritant or foreign material, an autoimmune disorder, a defect in the cells
responsible for mediating inflammation, recurrent episodes of acute
inflammation, and inflammatory and biochemical inducers that cause oxidative stress
and mitochondrial dysfunction.
Untreated injuries or infections can cause
chronic inflammation, which can be persistent or recurring. Chronic inflammation can result from exposure
to substances or industrial toxins that cannot be removed by enzymatic
breakdown or phagocytosis in the body.
In autoimmune illnesses, the immune system misidentifies typical bodily
components as foreign antigens and assaults healthy tissue, resulting in
diseases like rheumatoid arthritis and systemic lupus erythematosus. Certain
lifestyle variables can increase an individual's risk of developing chronic
inflammation. These include stress, smoking, inactivity, and a bad diet. Aging
can raise the risk of chronic inflammation, possibly as a result of a lifetime
of exposure to pollutants and poisons, or as a result of an increase in
visceral (belly) fat.
b) Strategies
for Early Diagnosis, Prevention, and Treatment of SCI
Early SCI diagnosis, prevention, and therapy
are critical for controlling the illness and avoiding severe health
consequences. For patients with
symptomatic SCI, magnetic resonance imaging (MRI) is suggested. It is the most
commonly used assessment to assess people with symptomatic SCI. Addressing
stress, eating a balanced diet, getting a massage or acupuncture treatment,
avoiding smoking and consuming alcohol in moderation, getting regular exercise,
and contemplating intermittent fasting are all prevention options. These measures
can aid in the reduction of inflammation and the prevention of SCI. Anti-inflammatory substances such as turmeric
and garlic can be used to treat chronic inflammation. However, long-term use of
NSAIDS and corticosteroids has been linked to health hazards, so it's critical
to check with a doctor before beginning any medication. In conclusion, knowing
the risk factors and applying measures for early diagnosis, prevention, and
treatment of SCI can dramatically lower the likelihood of acquiring chronic inflammation-related
disorders.
10) Systemic Inflammation in Specific Conditions
a) Chronic
Obstructive Pulmonary Disease (COPD)
Chronic obstructive pulmonary disease (COPD) is
a leading source of morbidity and mortality around the world. It is
distinguished by a weakly reversible airflow limitation that is typically
progressive and associated with an aberrant inflammatory response of the lungs
to noxious particles or gases, most notably cigarette smoke.
Low-grade systemic inflammation is thought to
be a hallmark of COPD and one of the primary processes responsible for the
increased prevalence of comorbidities, including cardiovascular problems. This
systemic inflammation is defined by a two- to fourfold rise in proinflammatory
and anti-inflammatory cytokines, naturally occurring cytokine antagonists,
acute phase proteins, and small elevations in neutrophil and natural killer
cell numbers.
The cause of systemic inflammation in COPD is
unknown. Some argue that local inflammation in the pulmonary compartment
spreads into the circulation, whereas others argue that non-pulmonary
compartments produce more inflammatory mediators.
b) Community
Acquired Pneumonia (CAP)
Community-acquired pneumonia (CAP) is a lung
parenchymal infection that causes significant mortality and morbidity
worldwide. Evaluating the systemic inflammatory response (SIR) in CAP may aid
in determining the etiological etiology as well as assessing the clinical
course, including therapy failure and prognosis.
C-reactive protein (CRP), a non-specific acute
phase protein generated by the liver in response to IL-6 stimulation, is
regarded as an important serum biomarker for SIR in CAP. Patients with CAP and
COPD had a lower inflammatory response than those without COPD, which is only
partially attributable to corticosteroid treatment.
Tobacco use appears to make people more
susceptible to CAP through a variety of processes that encourage respiratory
infection by inhibiting natural defensive systems. The rise in cellular
oxidative stress, which appears to elicit various responses to pathogens by
immune cells, including alveolar macrophages and peripheral blood mononuclear
cells, is crucial to this.
Finally, both COPD and CAP are linked to
systemic inflammation, which can have a substantial impact on the course and
prognosis of both diseases. Understanding the underlying processes of this
inflammation can aid in the development of successful therapeutic options.
11) Real Life
Testimonials and Stories
Ali Berger, 27, was diagnosed with rheumatoid
arthritis (RA) in November 2012, after completing numerous tests and doctor's
appointments. Rheumatoid arthritis is a chronic inflammatory condition that
affects more than just the joints. It can cause discomfort, stiffness, and
tiredness, making daily tasks difficult.
Despite the difficulties, Berger has been able
to properly manage her disease with the help of her family and medical team.
She decided to return to her hometown of Chicago from New York City in order to
find a rheumatologist closer to her family. Dr. Eric Ruderman, her
rheumatologist, was instrumental in developing an effective treatment plan for
her.
Berger has now been a supporter of the
rheumatic Research Foundation, working with the organization to underscore the
necessity of funding essential rheumatic research and developing a more strong
workforce to give patients across the country with improved access to
treatment.
Berger, like many others living with rheumatic
disease, had additional hurdles with the introduction of COVID-19. Many
rheumatologists have turned to telemedicine, which allows patients to consult
with a rheumatologist from a safe and secure distance. Berger has been able to
remain safe in her home while communicating online with Dr. Ruderman.
Berger has remained active and engaged in her
daily life despite the hurdles. "This is the most active I've ever been
since I was diagnosed," she boasts. I go for daily walks outside and play
tennis once a week..."I'm grateful that I'm healthy enough to do it."
This statement displays her tenacity and determination in dealing with her
illness.
Finally, Ali Berger's rheumatoid arthritis
experience highlights the value of a supportive medical team and family, the
possibilities of telemedicine, and the power of personal perseverance in
managing chronic diseases. Her experience can serve as an example to others who
are coping with similar health issues.
Jamie Stelter was diagnosed with rheumatoid
arthritis (RA) in 2003, a chronic inflammatory illness affecting the joints and
causing pain, edema, and stiffness. Her RA symptoms began with swollen
knuckles, and as time passed, most of her fingers grew deformed or bloated, and
she lost the ability to bend her wrists. Despite his physical limitations,
Stelter has managed to live a busy and full life.
Stelter works as a morning traffic reporter for
NY1 in New York City. She also has a food blog called TV Dinner, where she
shares healthy recipes and her RA experiences. In addition to her professional
endeavors, Stelter has written an e-novel called Transit Girl.
Stelter tried numerous drugs to control her RA
before settling on the one that worked best for her. She has also had neck and
foot fusion procedures, which have eased her pain and allowed her to maintain
her active lifestyle.
Stelter's approach to RA management goes beyond
conventional therapy. She has changed her lifestyle significantly, including
dietary changes and regular exercise. Her acupuncturist prescribed a vegan diet
at one time, which seemed to ease her issues. She has recently started eating a
Paleo diet. Stelter also makes time for rest and exercise, attending spin or
barre sessions three to five days each week.
Stelter's RA path has not been without
difficulties. Due to a total ankle replacement that permanently removed some of
the flexion in her foot, she has had to give up wearing high heels. She has,
however, adapted to these adjustments and is always seen wearing flats or
sneakers.
Despite the physical hurdles and lifestyle
changes, Stelter is optimistic and resilient. She sees her illness as something
she must live with and manage, rather than something that governs her life. Her
experience demonstrates the power of perseverance, optimism, and a proactive
approach to managing a chronic ailment like RA.
Mary's journey with Systemic Lupus
Erythematosus (SLE) began in 1995 and has been both difficult and uplifting.
Living with lupus is a lifelong commitment that necessitates continual
management and care, but Mary has managed to retain an active lifestyle and become
a champion of the Rheumatology Research Foundation despite these challenges.
Mary was diagnosed after experiencing a slew of strange symptoms, including
bruising, swollen and painful joints, persistent weariness, nausea, cold
sensitivity, mouth and nose ulcers, disorientation, and repeated infections.
She also got a 'butterfly' rash on her face, which is a frequent lupus sign.
The diagnosis was a relief, but it was also frightening and overwhelming. Her
early diagnosis and treatment, however, were critical in sparing her kidneys
and her life. Mary's experience with lupus has been a difficult one. She has
had to deal with new symptoms and therapies over the years because the
condition is unexpected. She has, however, kept an active lifestyle and has become
an active participant in her own self-care. She has also worked hard to put
together a multifaceted care team of health specialists who are familiar with
the numerous facets of lupus. Mary has become a strong supporter of the
rheumatic Research Foundation, highlighting the necessity of funding essential
rheumatic research and developing a more robust workforce to improve treatment
access for individuals across the country. Mary's work with the Foundation has
been crucial in achieving the Foundation's purpose of advancing research and
training to improve the health of persons living with rheumatic disease. Mary's
lupus journey has taught her the value of being an active participant in her
own self-care. She has learnt that living well with lupus is feasible, and that
people can help cope with the physical and mental effects of the disease by
being active participants in their own self-care. She has also learned the
value of having a support system comprised of people she can turn to for
assistance.
Amaka's battle with Systemic Lupus
Erythematosus (SLE) began in 2000. SLE is an autoimmune illness in which the
immune system attacks the body, resulting in a wide range of clinical symptoms
ranging from skin disorders to multi-organ systemic involvement. Amaka's
experience with the disease has been unpredictable, with periods of good health
followed by unexpected flares, a common feature of SLE.
Amaka's cancer diagnosis was a watershed moment
in her life. She was in the hospital for about a month before her illness was
determined. The medical staff described her diagnosis in detail, but the most
impactful message was that there was no cure for SLE. Despite the gravity of
the news, Amaka felt relieved that her symptoms were not imagined and that her
ailment had a name.
Following her diagnosis, Amaka was resolved to
live her life as normally as possible. She negotiated with her medical staff to
be allowed to attend university, agreeing to follow a stringent drug regimen
and regular hospital check-ups. She also pledged to leave university if she
shown any signs of a flare.
Amaka's symptoms worsened throughout her third
year of university. She chose to ignore these symptoms until she finished her
examinations, which culminated in a three-and-a-half-month hospital stay,
including time in intensive care and an operation to drain fluid from around
her heart. This incident showed her the significance of not neglecting her
symptoms, even while she was not experiencing a flare. She learnt to take care
of herself and avoid triggers that could cause a flare.
Amaka's path with SLE has not been easy, but
she has managed to retain a good attitude. She has gotten support from her
medical staff and other lupus patients. She has also benefited from
self-management strategies, which have been demonstrated to lower pain,
increase function, and delay impairment in lupus patients. These therapies
frequently include social support and health education, which can be very
beneficial for patients such Amaka
Amaka's story exemplifies the strength and
dedication of people living with SLE. Despite the difficulties, she has managed
to live a full life while managing her disability. Her story serves as an
example to others living with SLE and emphasizes the necessity of a supportive
medical team, self-care, and a good attitude in managing this chronic disease.
Sue's MS adventure began in 2002, when she was
referred to a neurologist after her urologist couldn't identify a physiological
basis for her recurring UTIs. The neurologist suspected MS and had an MRI of
her brain and spine performed. Sue's MS was verified by an MRI and a lumbar
puncture. Sue was taken aback by the diagnosis, as she had assumed she had a
disc condition. Despite seeking a second opinion, the diagnosis was confirmed.
The physicians also disclosed that she had MS for at least 20 years, which explained
her years of tripping, weariness, heat sensitivity, eye issues, frequent UTIs,
and numbness.
Sue was enraged by the diagnosis. She had led a
healthy lifestyle and had goals for the future that did not include being bound
by an illness. Her spouse, on the other hand, was more accepting of the
diagnosis and became her strongest supporter. He assisted her in realizing that
MS could not bind them unless they allowed it to.
Sue struggled to accept her diagnosis and the
knowledge that she had a condition for which there was no cure. She also
grappled with the reality that she was not as self-sufficient as she would want
and that she required assistance. She also recognized that, while her future
might not be what she had hoped for, it could still be rewarding.
Sue made the decision in 2016 to join a
neurology clinic that specialized in MS. She felt that her general practitioner
seemed unconcerned about what she was going through beyond the physical
symptoms. Dr. Katz at the Elliot Lewis Center assisted her in embracing
acceptance and understanding that she needed to ensure that the procedure did
not produce obstacles.
Sue had to make a difficult decision about when
to retire. Her job was becoming more demanding, which was affecting her MS. She
retired in March 2021, over twenty years after her diagnosis, and was ready to
embark on the next chapter of her life with MS.
Sue reflects back on the day she found out she
had MS and realizes that the life she imagined is still possible, just in a
different way. She has completed her master's degree, danced at her kids'
weddings, traveled with her spouse, and had a successful job since her
diagnosis. She is looking forward to the next chapter of her life, knowing that
there will be obstacles ahead, but she is a member of her treatment team, her
voice is heard and acknowledged, and they will get through it together.
Sue's experience demonstrates the significance
of being involved in one's own healthcare, asking questions, and seeking help.
It also emphasizes the significance of acceptance and the ability to adjust to
changing situations. Her experience with MS serves as a poignant reminder that,
while the condition may impose restrictions, it does not define who she is or
what she is capable of.
Jenna Green's MS journey began when a vehicle
accident left her in chronic pain and with unknown symptoms. She initially
blamed the symptoms on the accident, but when they persisted, she sought
medical attention. At the age of 31, she was diagnosed with relapsing-remitting
MS after a battery of tests and consultations. This type of MS is distinguished
by symptom flare-ups followed by periods of remission.
Despite her diagnosis's hurdles, Jenna found
strength in focusing on one task at a time, a departure from her former
multitasking lifestyle. She also realized the importance of sharing her journey
on social media platforms such as Instagram, where she increases MS awareness
and advocates for those affected by the disease.
Jenna's activism goes beyond social media. She
has testified in the Massachusetts State House on multiple occasions in support
of laws affecting individuals with MS. She also serves on the government
relations committee for the National Multiple Sclerosis Society. Her advocacy
efforts have resulted in substantial changes, such as increased funding for MS
research and continued support for the Centers for Disease Control and
Prevention's National Neurological Conditions Surveillance System.
Jenna's MS journey has also led her to
investigate other therapy possibilities. To manage her symptoms, she used a
combination of Western medicine, natural healing therapies, and thinking
exercises. She also discovered that getting the correct medication was critical
to controlling her symptoms. However, she encountered difficulties when her
insurance company refused to cover the necessary medication, prompting her to
become an advocate for herself and others in similar situations.
Jenna now owns a strategic marketing consulting
firm, Full of Grit and Grace, in addition to her advocacy efforts. She uses her
storytelling abilities to make a difference, and she just received a
HealtheVoices Impact Fund award to develop a series of YouTube videos to help
people with chronic conditions get more comfortable with public speaking.
Despite her difficulties, Jenna is optimistic
about her future. She feels that telling her experience will help her reclaim
her power and battle the stigma connected with diseases like MS. She continues
to increase MS awareness and fight for individuals affected by the disease,
exhibiting tenacity and determination in the face of adversity.
12) Conclusion
To summarize, systemic inflammation is a
complex biological immunological response that can be induced by a range of
reasons such as infections, damaged cells, and toxic substances. Chronic
inflammation, in particular, is a sluggish, long-term inflammation that lasts
for several months to years. Failure to eliminate the agent causing acute
inflammation, exposure to a low level of a specific irritant, an autoimmune
disorder, a defect in the cells responsible for mediating inflammation,
recurrent episodes of acute inflammation, and inflammatory and biochemical
inducers causing oxidative stress and mitochondrial dysfunction are all
possible causes.
Chronic inflammatory diseases are a major cause
of death worldwide, with chronic inflammation being connected to problems such
as stroke, chronic respiratory diseases, heart disorders, cancer, obesity, and
diabetes. It's also linked to rheumatoid arthritis, systemic lupus
erythematosus, and other disorders.
It is crucial to emphasize, however, that
inflammation is not inherently harmful. Acute inflammation is an important
aspect of the body's defensive mechanism since it recognizes and removes
harmful and foreign stimuli while also commencing the healing process. The
issue emerges when inflammation becomes chronic and uncontrolled, potentially
causing tissue damage or disease.
Managing chronic inflammation frequently
entails lifestyle adjustments such as keeping a healthy weight, eating a
nutritious diet, getting plenty of rest, and exercising regularly. Certain
nutrients are connected to either boosting or suppressing the inflammatory
response, and regular exercise can help protect against chronic
inflammation-related illnesses.
Finally, knowing the mechanics of chronic
inflammation and its consequences for human health is critical. It enables us
to take preventive measures to manage our lifestyle and food, potentially
lowering our risk of chronic diseases connected with inflammation. It serves as
a reminder that our daily actions have a big impact on our long-term health.
1) What is systemic inflammation?
Systemic inflammation is a condition where the body's immune system is constantly triggered, causing damage to healthy cells and tissues. It is especially problematic when the body reacts as if it were injured or sick, even when there's nothing to fight
2) What causes systemic inflammation?
Many conditions can cause systemic inflammation, including type 1 or type 2 diabetes, gout, and rheumatoid arthritis. Unhealthy habits such as poor diet, smoking, and obesity can also contribute to chronic inflammation
3) What are the symptoms of systemic inflammation?
Symptoms can include fever, weight loss, low energy levels, skin rashes, and swollen and painful joints
4) Can systemic inflammation be cured?
The possibility of a cure depends on the cause. Chronic inflammation is typically a sign of an underlying condition. For some conditions like gout, lifestyle changes and medications can reduce inflammation. However, conditions like rheumatoid arthritis or systemic lupus usually require long-term medications
5) How is systemic inflammation treated?
Treatment depends on the cause. It could involve dietary changes, medications, or disease-specific treatments. For instance, chronic hepatitis might require antiviral treatment, while rheumatoid arthritis or systemic lupus could be treated with immune-suppressing medications
6) Does drinking lots of water help with inflammation?
Yes, drinking water regularly helps your kidneys function normally, which can help flush toxins out of your body. This can reduce inflammation because toxins in your body can trigger inflammation
7) Is there a test for chronic inflammation?
Yes, there are blood tests that can show how much inflammation you have in your body. However, these tests do not necessarily indicate the cause of the inflammation
8) What foods reduce inflammation in the body?
Adjusting your diet can potentially help reduce the amount of chronic inflammation in your body. Avoid highly processed foods and foods with high amounts of sugar or sweeteners. Increase the amount of fruits, vegetables, and whole grains that you eat
Proinflammatory mediators play a crucial role in the pathophysiology of SIRS. They are responsible for initiating and propagating the inflammatory response, which can lead to tissue damage and organ dysfunction
Coagulation plays a significant role in the pathogenesis of SIRS. It can lead to the formation of blood clots, which can obstruct blood flow and contribute to organ dysfunction
11) What are the diagnostic criteria for systemic inflammatory response syndrome (SIRS)?
The diagnostic criteria for SIRS include body temperature abnormalities, heart rate abnormalities, respiratory rate abnormalities, and abnormal white blood cell count
12) What is the role of antibiotic therapy for systemic inflammatory response syndrome (SIRS)?
Antibiotic therapy is crucial in the treatment of SIRS when it is caused by a bacterial infection. Broad-spectrum antibiotics are often used initially until the specific causative bacteria are identified
Proper nutrition plays a vital role in the treatment of SIRS. It helps to support the immune system, promote healing, and prevent malnutrition, which can worsen the condition
14) What is the focus of treatment for systemic inflammatory response syndrome (SIRS)?
The focus of treatment for SIRS is to control the underlying cause of the inflammation, manage symptoms, and prevent complications
Increased levels of interleukin 6 (IL-6), a pro-inflammatory cytokine, can indicate an ongoing inflammatory response in SIRS. It can be used as a marker of disease severity
Imaging studies can help identify the source of infection or inflammation in SIRS, aiding in diagnosis and treatment planning
Supplemental oxygen is often used in the treatment of SIRS to improve oxygenation and prevent organ damage due to hypoxia
Vasopressin can be used in the treatment of SIRS to increase blood pressure and improve organ perfusion, especially in cases where the condition has led to septic shock
ACTH stimulation testing can be used in the management of SIRS to assess adrenal function, as adrenal insufficiency can occur in severe cases of SIRS
The type of specialist needed for the treatment of SIRS depends on the underlying cause. It could involve consultations with infectious disease specialists, rheumatologists, endocrinologists, or other specialists as needed


.jpg)







Comments
Post a Comment